This year's Gaokao chemistry question from Hubei is quite interesting...
Release time:2025-07-04
In the 2025 Hubei Gaokao (College Entrance Examination) chemistry paper, a question about the "lithium replenishment mechanism of lithium bis(trifluoromethanesulfonyl)imide" stumped some candidates.

Question 12 of the 2025 Gaokao Chemistry Paper
The chemistry examiners cleverly incorporated the "battery injection" technology, which went viral in February this year thanks to a Fudan University team, into this seemingly complex electrochemical examination point.
It's clear that the national team attaches great importance to lithium replenishment technology.
This question fully presents the core principles of lithium-ion battery lithium replenishment technology, involving materials, electrochemistry, and engineering applications, especially reflecting "Materials - Reaction - Performance" integrated design ideas.

Analyzing the knowledge points of this Gaokao chemistry question from the perspective of battery research and development, the summary is as follows:
01. Battery Materials and Structure
Cathode Material: LiFePO₄ (lithium iron phosphate), commonly used in lithium-ion batteries, has high safety and stability.
Anode Material: Graphite intercalated with lithium (Li⁺ embedded between graphite layers), a typical anode material for lithium-ion batteries.
Lithium Replenishment Reagent: LiSO₂CF₃ (lithium bis(trifluoromethanesulfonyl)imide derivative), which is injected into the battery to regenerate deactivated batteries while maintaining the original structure. It is a key material in battery repair technology.
Supplement: The Fudan University team also used AI-assisted molecular design to screen this new lithium carrier from 240 candidate molecules and experimentally verified its ability to achieve "non-invasive lithium replenishment" of the battery.
02. Electrochemical Process
Anode Reaction: [SO₂CF₃]⁻ loses electrons at the anode to generate SO₂ and fluorocarbons (such as CF₃-CF₃). The reaction formula is:
2[SO₂CF₃]⁻ - 2e⁻ → 2SO₂↑ + CF₃-CF₃↑
This process releases Li⁺, replenishing the lithium loss at the anode.
Cathode Reaction: Li⁺ is re-embedded into the graphite anode at the cathode, restoring the battery capacity.
Key Advantages: After the reaction, the lithium replenishment reagent leaves in the form of gas, without damaging the original structure of the battery. This solves the problem of traditional lithium replenishment requiring battery disassembly and is a battery life extension technology.
03. Core Issues in Battery Research and Development
Lithium Loss Repair: Addressing the irreversible loss of lithium during battery cycling, the lithium replenishment reagent is used to supplement Li⁺, improving the reversible capacity of the battery.
Material Stability: During the lithium replenishment process, the iron element valence state of the LiFePO₄ cathode remains unchanged (+2), avoiding structural damage and demonstrating the stability of the material design.
Environmental Protection: The byproducts are SO₂ and fluorocarbons (non-toxic gases), avoiding the pollution problems of traditional lithium replenishment reagents and aligning with green chemistry principles.
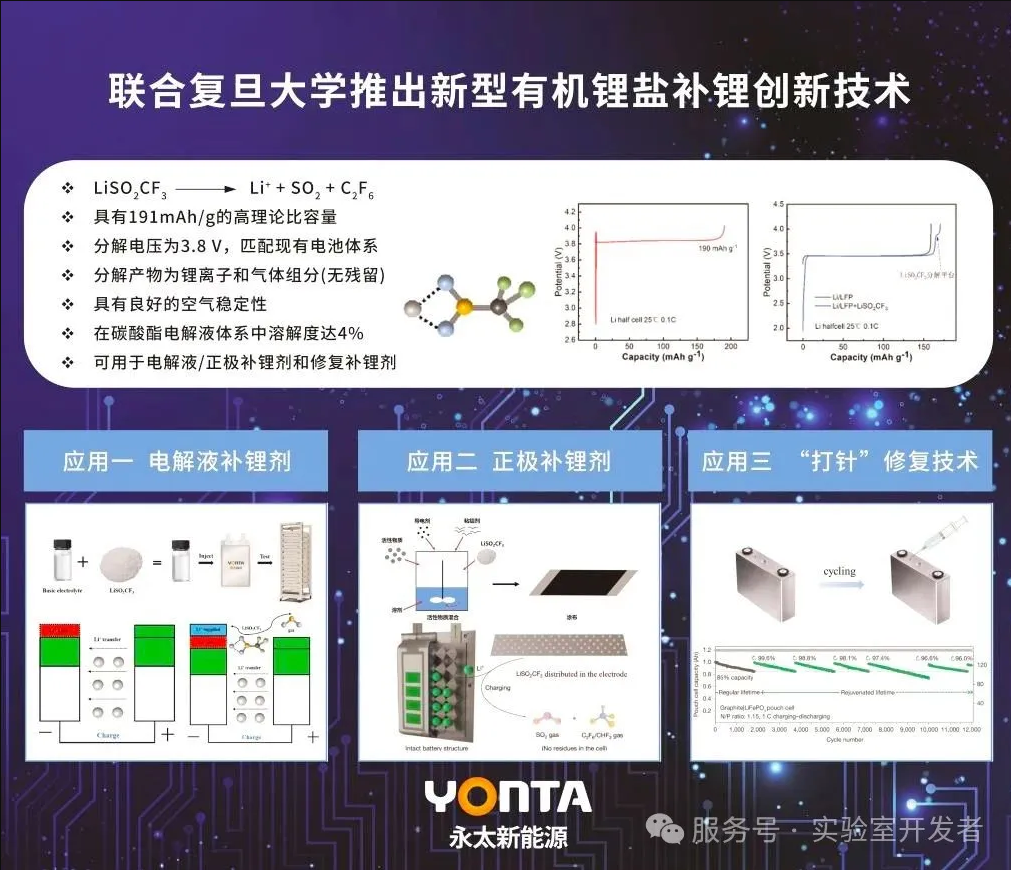
Image source from the internet, please delete if infringement.
This achievement by the Fudan University team was published in Nature under the title "External Li supply reshapes Li-deficiency and lifetime limit of batteries".
It is worth mentioning that the team successfully combined AI to build a chemical database, using unsupervised machine learning for molecular recommendation and prediction, successfully obtaining a previously unreported lithium carrier molecule—lithium trifluoromethylsulfinate (CF3SO2Li), making the AI for Science concept truly come to fruition.
The Hubei Gaokao chemistry examiner's intention was extremely meticulous, and the concept of the lithium replenishment reagent screened by artificial intelligence AI is also indicated in the question.
Stepping outside textbooks and laboratories, let's talk about the specific impact this new technology will have on our lives.
Electric vehicles losing range after a few years, mobile phone batteries bulging after half a year—these headaches are rooted in the fact that the lithium element in the battery slowly "disappears." In traditional lithium batteries, lithium is irreversibly consumed during charging and discharging, such as forming SEI films and embedding inert substances, like the "battery memory effect" where the phone battery never fully charges.
The lithium bis(trifluoromethanesulfonyl)imide mentioned in the exam question The magic of the lithium replenishment agent lies in its "non-invasive repair": unlike traditional methods, it doesn't require disassembling the battery. Simply inject this reagent into the battery, and it will react at the anode, releasing lithium ions that are re-embedded into the graphite layer of the cathode.
Once the technology becomes widespread, "buying a car that can be driven for a lifetime" may not be difficult to achieve.
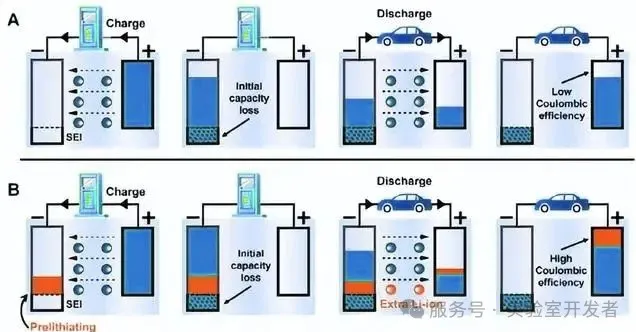
(A) Schematic diagram of the effect of initial capacity decay on battery cycle capacity (B) Schematic diagram of battery cycle capacity improvement by lithium replenishment technology. Image source from the internet, please delete if infringement.
The changes brought about by lithium replenishment technology are not limited to improved range.
Globally, 50 million tons of e-waste are generated annually, half of which is discarded batteries. Lithium replenishment technology extends battery life and reduces repair costs, effectively reducing battery waste from the source.
Lithium resource consumption will also decrease significantly, and lithium replenishment technology can reduce the demand for native lithium for batteries.
You might think these changes are still far off, but let's look at the current dynamics of the energy industry.
In the first quarter of 2025, funding in the lithium replenishment agent field surged, and leading battery R&D companies are stepping up efforts to build factories;
The Ministry of Industry and Information Technology has explicitly included lithium replenishment technology in the "14th Five-Year Plan" key research projects, providing a subsidy of 0.3 yuan per watt-hour for energy storage projects using lithium replenishment agents.

Changes in the battery market caused by the implementation of lithium replenishment technology
The emergence of lithium replenishment technology is merely a microcosm of the new energy revolution in 2025.
While we complain about the short lifespan of batteries, electrochemical scientists are dissecting the movement of each atom in the laboratory;
While we look forward to longer battery life, battery R&D engineers are testing the implementation of lithium replenishment technology on the production line.
It is hoped that this great technology of lithium replenishment will be put into practical application—mobile phones will last longer, electric vehicles will travel further, electricity bills will be thinner, and even our planet will be greener due to less battery waste.
To date, the college entrance examination scores for various provinces in 2025 have been released. We hope that all candidates can enter their ideal universities, and those interested in battery and new energy research can also consider applying for electrochemistry-related majors.
Let us also look forward to 2026 What disruptive battery technology will be included in The college entrance examination chemistry syllabus Scope?
END
Disclaimer: Some of the content and materials (including images) in this article are reproduced from online sources. This article does not represent the views of this platform and is for learning and exchange purposes only. It does not constitute commercial purposes, and the copyright belongs to the original author. If there are any disputes regarding the content, copyright, and other issues, please contact us via email cj017@spcmach.com. This platform will verify and reply within 24 hours. This article may not be reproduced without authorization. We will not bear any legal responsibility for any consequences caused by unauthorized reproduction.
Hubei College Entrance Examination Chemistry 2025,Lithium replenishment technology,Fudan University lithium supplementation technology,College entrance examination chemistry questions,Renewable energy technology
Related News
Focus on us



