Understanding in One Reading | From Lab to Production Line: An Analysis of Dry/Wet Pulp Processes for Electrodes
Release time:2025-05-28
70% of a battery's performance is determined by the electrode slurry, and the core objective of the slurry preparation process is to achieve "uniform dispersion of three phases" - with changes in temperature, viscosity, and environment, Active material (Energy storage main body, responsible for storing charge, such as lithium iron phosphate in the positive electrode and graphite in the negative electrode), Conductive agent (Charge pathway, such as carbon nanotubes), Binder (Structural support, such as PVDF) in Solvent (Mixed into slurry, such as NMP) to form a stable suspension, which is finally coated into an electrode with an ideal porous structure.
This article interprets the two major processes of battery slurry preparation: "dry slurry preparation" and "wet slurry preparation." What are the core differences in their principles?
Which slurry preparation process is more suitable for your project?
How to balance cost and efficiency?
Is the technical barrier high? Helping you deal with project decisions!
Ideal electrode characteristics
① Active material particles are uniformly dispersed without agglomeration;
② The conductive agent forms a continuous conductive network, wrapping the active material particles;
③ The binder is uniformly distributed, balancing mechanical strength and ion channel retention.
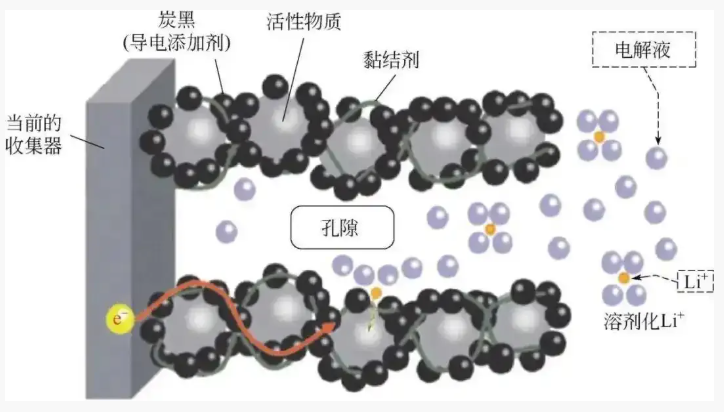
Ideal distribution state of various materials in lithium-ion battery electrodes: Image source network, infringement deletion
WET COATING | Wet slurry preparation process : Sol first, then mixing
Core principle: Using the solvent as a medium, a uniform slurry is constructed through a three-step method of "dissolution - dispersion - stabilization." The core relies on the binder's solvation and shear force to assist dispersion.
01. Wet slurry preparation core process
| Steps | Popular understanding | Time consumption | Key equipment |
| Sol | Soak the binder and stir it into a "glue"-like state | 30-60min | Low-speed mixer |
| Glue preparation | Add "conductive agent" and stir to form "conductive glue" | 20-30min | High-speed disperser |
| Glue mixing | Pour in "active material fragments" (such as lithium iron phosphate) | 60-90min | Planetary mixer |
| Viscosity adjustment | Add/reduce the ratio to adjust to a viscosity suitable for coating | 10-20min | Viscometer |
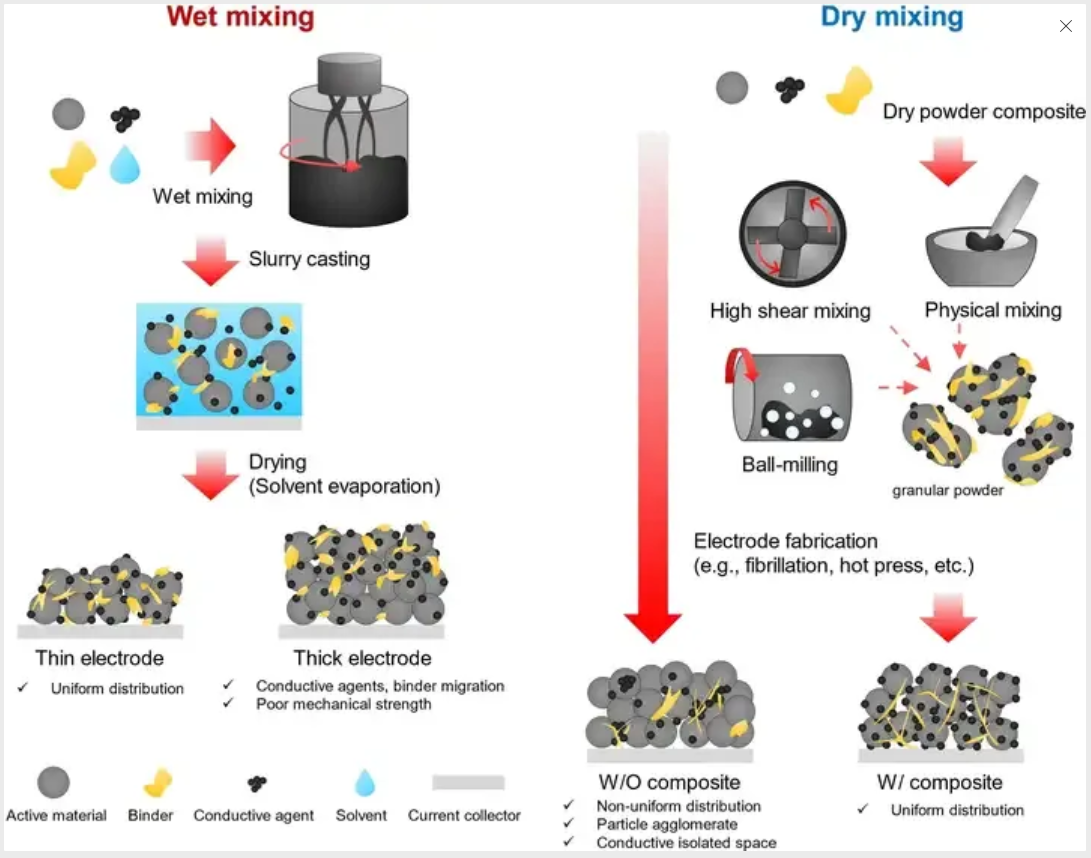
Comparison diagram of wet and dry electrode mixing processes: Image source network, infringement deletion
0 2. Dispersion principle
Solving particle "agglomeration" Particle size effect Micrometer-level active materials (such as NCM811, D50=10-15μm) need to be broken down to 5-10μm, and nanometer-level conductive agents (such as carbon black, D50=50nm) need to prevent agglomeration into aggregates larger than 100nm.
Key issue: Small particles have a large specific surface area (e.g., the specific surface area of lithium iron phosphate > 10m²/g), and are easily formed into soft agglomerates due to van der Waals forces. This requires forced dispersion through shear force (high-speed stirring above 5000rpm) + surfactant (such as CMC).
03. Slurry stability
Preventing "layering and sedimentation" Optimal viscosity range: 5000-15000mPa・s (adjusted according to the coating method, the comma scraper needs to be lower, and the die head coating needs to be higher).
Core formula: Viscosity = (Solid content × Particle specific surface area) / (Solvent fluidity × Binder molecular weight). The viscosity of the NMP solvent system is 30%-50% higher than that of the water system.
Binder function PVDF: Through polar groups (-CF2-) to adsorb active materials (such as NCM surface hydroxyl groups), forming a physical cross-linking network. The molecular weight needs to be > 500,000 to ensure bonding strength (peel strength > 30N/m).
CMC/SBR (commonly used in negative electrodes): CMC provides electrostatic repulsion (negative charge groups), and SBR provides an elastic network, synergistically inhibiting the shedding of graphite particles (expansion rate 12%).
Slurry stored for more than 48 hours is prone to layering due to gravity causing the conductive agent to sink (density difference > 1g/cm³). Anti-settling agents (such as fumed silica) or regular stirring are required.
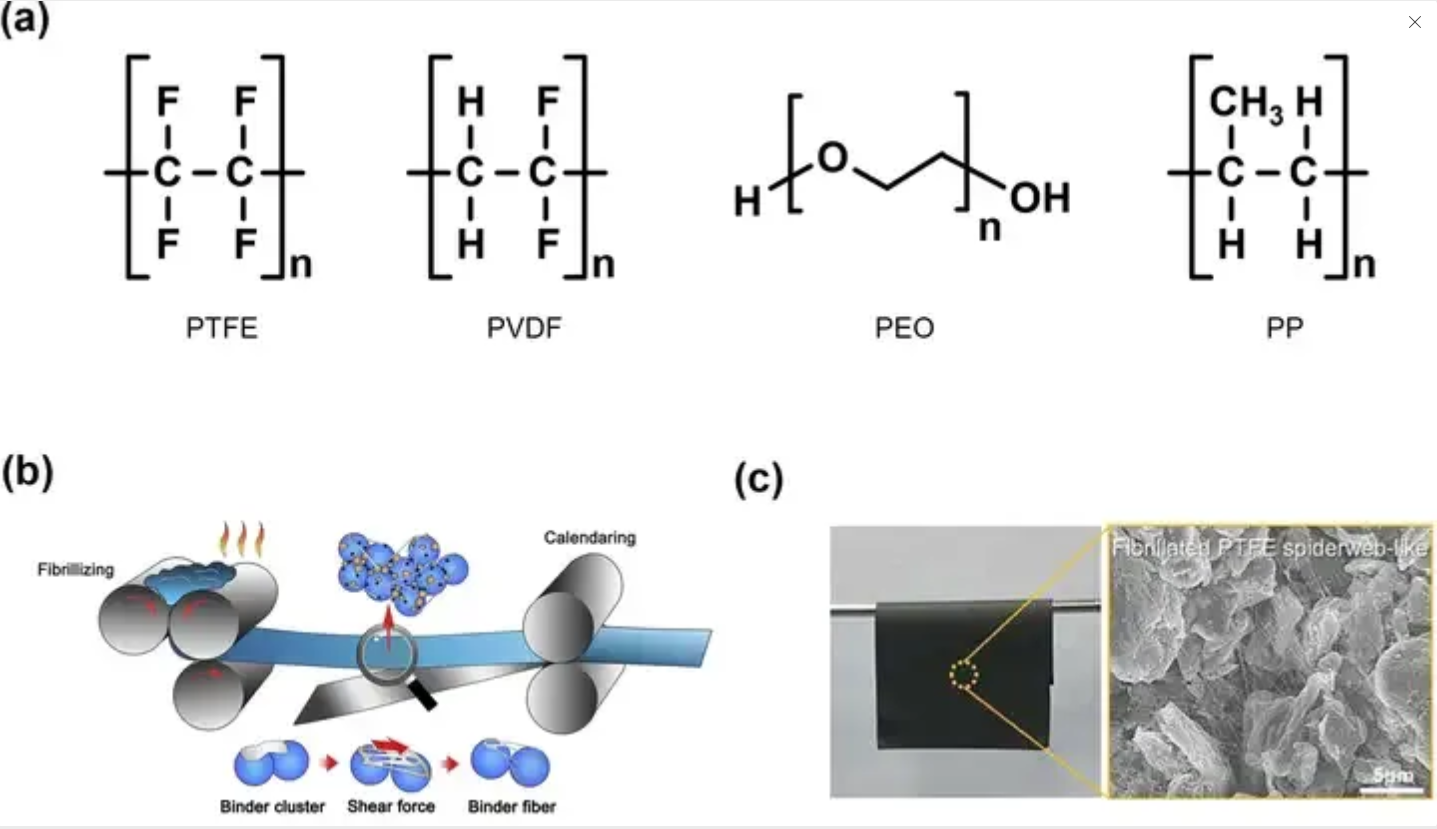
Copyright (2023)Springer: Dry electrode binder characteristics, (a) PTFE as a representative polymer used as a dry electrode binder, (b) Typical steps in manufacturing dry electrodes through binder fibrillation. (c) Electrode production and its SEM image through PTFE fibrillation, image source network, infringement deletion
DRY COATING | Dry slurry preparation process: Mix dry powder first, then knead evenly
Core principle: Under solvent-free conditions, through High shear force Breaking up agglomerates in dry powder, relying on binder fibrillation to form a physical network, achieving "dry powder mixing - solvent wetting - gradient dispersion"
01 Core process of dry method pulping
Directly pour the dry powder of active material, conductive agent, and binder together and stir at high speed to break up large agglomerates;
Add solvent several times, and after each addition, stir at high speed shear to force the solvent into the gaps in the dry powder, forcing them to disperse evenly to form a high-concentration slurry.
| Steps | Time consumption | Key equipment |
| Dry powder blending | 60-120min | High shear mixer |
| Solvent wetting | 30-40min | Same as above |
| Gradient dilution | 40-60min | Ultrasonic disperser |

(Dry method battery electrode preparation process: Picture source network, delete if infringement)
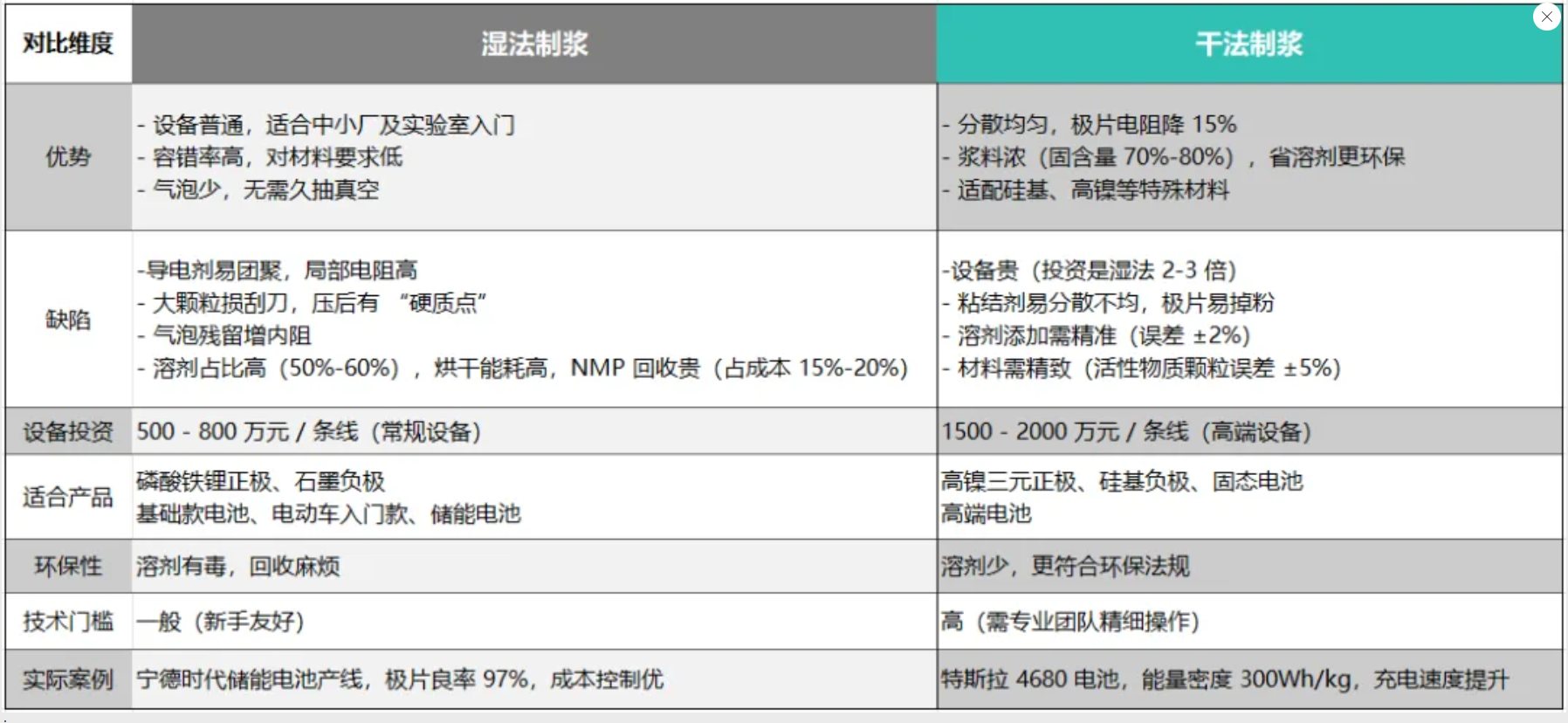
Comparison of dry and wet method applications
There is no best process, only the most suitable choice.
Dry pulping and wet pulping are like the "left and right legs" of new energy batteries:
-
If you are a "pragmatist" who pursues cost-effectiveness, wet method can help you control costs and handle conventional products;
-
If you are an "innovative" aiming at the high-end, the dry method will become your key weapon to break through performance bottlenecks.
For R&D personnel, it is necessary to cultivate the correlation mechanism of "shear force - molecular conformation - interface performance";
For enterprises, they should choose "wet method stability" or "dry method innovation" according to product positioning.
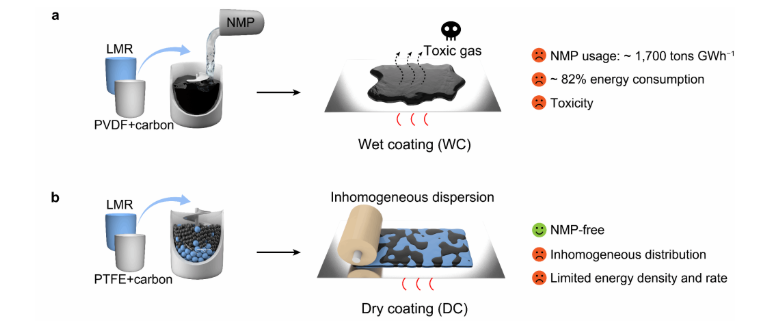
Image source: Sun Hao team Advanced Materials different electrode preparation process characteristics
Chaodian Conclusion
With the advancement of material technology and the tightening of environmental protection policies, the importance of dry-method technology is becoming increasingly prominent. However, the current industry is still dominated by wet methods, as it is adaptable to mature materials and large-scale production. Even so, leading companies such as CATL have developed a "dry-wet mixed process" - first pre-dispersing the conductive agent by wet method, then secondary strengthening by dry method, the performance is close to pure dry method.
For new energy companies and R&D personnel, understanding the essential differences between the two and choosing or integrating processes according to their own needs is the key to success.
Every step of evolution in battery slurry preparation technology is a key bridge for new energy to move from the laboratory to large-scale application.
Core data sources and references of this article:
Kim, Y. et al. "Effect of shear rate on carbon nanotube dispersion in lithium-ion battery electrodes." Journal of The Electrochemical Society 168, 050513 (2021)
Tsinghua University team, "The influence of mechanical force-induced PTFE fibrillation on the interface stability of silicon-based anodes", Advanced Energy Materials 14, 2303892 (2024). MIT research: "Conductive network fractal dimension in lithium-ion battery electrodes", Joule 8, 1234-1248 (2024). Alkali-resistant binder R&D progress, Zhejiang University team, ACS Applied Materials & Interfaces 15, 32456-32465 (2024). Sun Hao team Advanced Materials: Phase change regulation strategy unlocks sustainable, high-performance dry electrodes.
Other parts of the content are collected from publicly available online resources.
👉Are you sticking to the wet method to protect the present, betting on the dry method to fight for the future, or looking for a breakthrough in the mixed process? Welcome to share practical experience
✋ Follow the Wuhan Chaodian Technology WeChat official account, send a private message to the background, and receive 《2025 China Solid-State Battery Technology Seminar Expert Report》.zip Data package (big shot ppt)
*Disclaimer*: The views in this article are from the Internet, not my own. Some materials (including pictures) are reproduced from online materials, and are only for learning and exchange, not for commercial purposes. The copyright belongs to the original author. Thank you to industry predecessors for their contributions. If there are any disputes regarding the content, copyright, and other issues, please contact us via email cj017@spcmach.com, and we will verify and reply within 24 hours. This article may not be reproduced without authorization. We will not bear any legal responsibility for any consequences caused by reproduction.
Dry electrode,Dry process,Solid-state electrolyte,Wet process,Battery slurry process
Previous Page
Previous Page
Related News
Focus on us



